R&D projects
The Peaches Group focuses its research efforts on its own Clinical Trials for Innovation in Regenerative Medicine with Universities, Hospitals and Public and Private Research Centers of international excellence to develop value-added products, technologies and services for cell therapy within the reach of different specialties. medical and beneficial for millions of patients with maximum safety and effectiveness.
PRS® CK STORM
Treatment and prevention of cytokine storm
A phase I/II clinical trial is being conducted at the Fuenlabrada University Hospital for the prevention and treatment of acute respiratory distress syndrome (ARDS) caused by the most prevalent viral aetiologies: COVID-19, influenza A, influenza B and respiratory syncytial virus (RSV).
Our drug will change the treatment paradigm for the cytokine storm associated with ARDS, a syndrome of great relevance in the healthcare system due to its high incidence in critical patients, the intensive use of resources (ICU, prolonged mechanical ventilation) and the impact on costs and mortality.
The product patented by Peaches Biotech is based on a cytokine concentrate derived from m2-type macrophages and adult mesenchymal stem cells extracted from adipose tissue (PRS® CK STORM).
The trial is being conducted at the Fuenlabrada University Hospital, led by Dr. Francesca Sarno as Associate Researcher at the Company and Dr. David Bernal as Principal Investigator at the Hospital Centre.
Promoted by the Peaches Group.
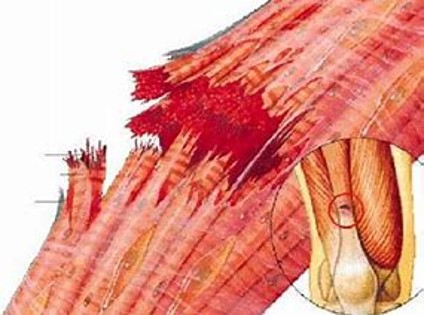
Mioboost y TenoBoost
Treatment for muscle regeneration
At the LIVING CELLS Bioincubator, research is advancing on allogeneic biological products for the regeneration of muscle and tendon tissue.
These products, PRS MioBoost and TenoBoost, developed under the PRS® (Selective Rich Plasma) patent (EP19382247.5) of PEACHES S.L., act through immunomodulation processes that induce muscle and tendon regeneration phenomena, respectively, which are especially beneficial for athletes with these types of injuries.
Forty-five per cent of the general population in Spain practises a sport, and 68 per cent of them suffer an injury each year (38 per cent ILT). In everyday life, 30 per cent of workers suffer from at least one tendinosis, and the increase in physical exercise among the population exponentially increases everyday injuries. In Europe, companies producing biological products for the treatment of sports injuries have a turnover of more than €500 million.
The PRS’s competitor in the market is PRP (Platelet-Rich Plasma), which is used in more than 45 million treatments worldwide.
PRS MioBoost and PRS TenoBoost base their effectiveness on cellular immunomodulation to stimulate the regeneration of injured tissues with selective growth factors.
The mechanism of action of these products is based on the same principles as PRP, but differs in that, while PRP is a universal product in which all types of growth factors are released by platelet lysis, PRS selects specific growth factors for the particular tissue to be regenerated, in this case muscle and tendon tissue, allowing for targeted treatment, which reduces the physiological recovery time of the injury by 50% compared to PRP, optimising the regeneration of the tissue in question and preventing fibrosis.
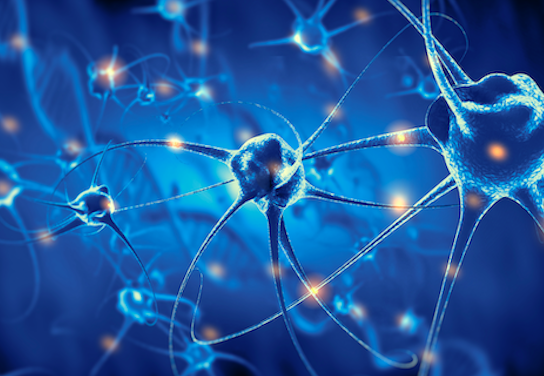
Neuropainboost
Treatment of Localised Neuropathic Pain with PRS® Microglial
The product under investigation, PRS NEUROPAIN, is designed to treat neuropathic pain, a nervous system disorder that occurs without any real threat.
This product, developed under the PRS® (Selective Plasma Rich) patent (EP19382247.5) of PEACHES S.L., acts through cellular immunomodulation processes, using selective growth factors to stimulate the regeneration of damaged nerve tissue.
The efficacy of PRS® Neuropain in the treatment of neuropathic pain is based on preventing the antigenic recognition of SAPS and DAMPS by neuronal microglia and restoring the normal functioning of the peripheral nervous system.
The clinical trial with the PRS Neuropain product will be led by Dr. David Abejón, Specialist in Anaesthesiology, Resuscitation and Pain Therapy, Full Member of the RAMON Y CAJAL Academy, Fellow of Interventional Pain Practice (FIPP), Winner of the 2018 Award for Excellence in Healthcare and International Opinion Leader in Pain Treatment.
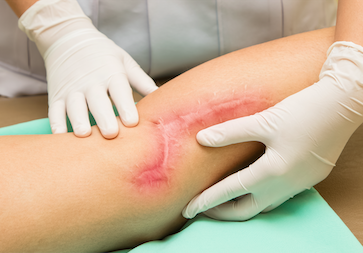
Scarboost
Optimization of Surgical Scars
SCARBOOST is an ambitious project currently in development that aims to optimise the healing process that occurs after a surgical wound. The procedure developed by Peaches is based on implanting 100 million expanded allogeneic ADSC cells adsorbed onto a hyaluronic acid carrier to achieve the stated objective of minimising surgical scarring.
This procedure is performed during the same surgical procedure through a simple local injection process during the closure of the surgical wound.
The ability to treat skin lesions so that they heal without fibrosis aims to revolutionise the care of lacerations, surgical incisions and burns, and will alleviate a great deal of suffering, with the obvious commercial impact that this will have as it can be applied to the extremely high number of procedures that are performed.
The trial is scheduled to take place at the Vall D’Hebron Hospital in Barcelona, led by the Head of the Plastic Surgery and Burns Department, Dr. Joan Barret.
Promoted by the Peaches Group.
OpTcells
Pancreatic cancer treatment – Personalised therapy with ‘Organoid Primer T cells’
Pancreatic ductal adenocarcinoma (PDAC) is a silent type of cancer that is incurable by the time it is diagnosed. Despite current drug treatments, the mortality rate for this type of tumour is very high.
The survival rate for patients diagnosed with DCP is less than 5%: only 5 out of every 100 people diagnosed survive after 5 years of treatment.
In recent years, the Madrid-based Immunoncology research group, in collaboration with researchers from Harvard Medical School in Boston and Weill Cornell University in New York, has been working on an immunological model based on educating the patient’s own immune defence cells to destroy the cancer cells in the pancreas.
One of the current strategies in immunotherapy is called adoptive cell therapy (ACT). This approach is based on using the patient’s own immune cells to attack the cancer.
The application of this highly innovative and promising approach to patients with pancreatic ductal adenocarcinoma could provide a paradigm shift and potentially prevent the recurrence of this highly fatal disease.
This technology is being developed in the LivingCells laboratory at the Bioincubator of the Fuenlabrada University Hospital, managed by the Peaches Biotech Group.
The Immunoncology research group is led by Dr Manuel Hidalgo, a world-renowned researcher in pancreatic cancer and a pioneer in the development of preclinical platforms used in translational cancer research. Currently, in vivo (Patient Derived Xenograft or Avatar model) and in vitro (Patient Derived Organoid or Organoid) models continue to be used for the development of biomarkers related to the detection of Pancreatic Ductal Adenocarcinoma and for personalised and precision medicine.
In this project, the objective is to generate autologous T cells from peripheral blood, which would target the patient’s own tumour. These lymphocytes are co-cultured with autologous cancer cells, in the presence of cytokines, to allow the activation and expansion of T cells and the destruction of tumour cells. The result of the lymphocyte amplification and activation processes would be opTcells (organoid primed T cells), which would have the ability to effectively destroy autologous tumour cells with high efficiency. The opT cells will be characterised to demonstrate that they express the activation of central memory markers.
The results in animals are showing very high efficacy.
The information obtained could be transferred to patients, adapting treatments and positively influencing the course of the disease.
Promoted by Grupo Peaches.

Granulisina
Treatment to destroy cancerous tumours
The GRANUILISINA project is being developed in collaboration between Peaches S.L. and the University of Zaragoza at the LIVING CELLS Bioincubator of the Fuenlabrada University Hospital, managed by the Peaches Group. It is based on the patent ‘GRANULISINA, METHOD OF OBTAINING AND USES (PCT/EP2019/069979)’ owned by the University of Zaragoza, for which Peaches S.L. has the exclusive licence. The aim is to achieve a highly effective treatment for cancerous tumours, particularly colon cancer.
The importance of this cancer lies in the fact that it has the highest incidence worldwide. In fact, one in 20 men and 30 women will suffer from colon cancer before the age of 70, with a 54% survival rate at 5 years. In economic terms, this problem represents an average annual cost per patient of €6,700.
The study is based on the use of Granulysin, a protein produced by cytolytic T lymphocytes and natural killer cells (or NK cells). We have verified its cytolytic activity against tumour cell lines in the laboratory, as it induces cell death through apoptosis. Studies conducted in in vivo models of tumour development (breast adenocarcinoma, multiple myeloma, melanoma) by intratumoural administration have already demonstrated its effectiveness, indicating that it may also have an activating effect on antitumour NK cells.
In a next step using this patent, the aim is to produce recombinant proteins resulting from the fusion of Granulysin with the corresponding antigen recognition fragments of antibodies that recognise tumour antigens. In this way, we will improve the directionality of Granulysin towards specific tumours to allow systemic treatment, which is much more suited to clinical use than intratumoural administration.
These studies are led by Professor Alberto Anel of the University of Zaragoza, who is obtaining positive results from in vivo experimentation in models of HT29 colon carcinoma and HeLa-CEA cervical tumour development in athymic mice. Peaches will carry out the industrial scaling and will subsequently promote the corresponding clinical trial.
Project CPP2021-008506 has been funded by MICIU/AEI/10.13039/501100011033 and by the European Union-NextGenerationEU/PRTR through the 2021 Public-Private Partnership programme call for proposals.

STA
Transfer and Nesting Solution
The R&D project, ‘Development of a Tissue-Specific Transfer Solution for the Optimization of Cell Nesting in Advanced Therapies’, developed by PEACHES S.L., has been co-financed by the European Regional Development Fund (ERDF 2021-2027) and the Centre for Industrial Technological Development (CDTI) with the aim of researching and developing new advanced therapy medicines that enable the regeneration, recovery or replacement of damaged tissues from biological material. The project had a budget of 703,628€.
Thanks to the support provided, PEACHES S.L. has carried out this R&D&I project, concluding that STA has advantages over PRP in terms of cytokine balance, stability and reproducibility. This represents a significant advance in the field of regenerative medicine, demonstrating the efficacy and safety of STA and providing a solid basis for future clinical applications in tissue regeneration and repair.

There is still a long way to go
For this reason, we continue to investigate in different fields such as:
CARDIOLOGY
LYMPHODEMA
LOCATION
C/ Isabel Colbrand 6
4ª Planta B
28050 Madrid (Spain)
OPENING HOURS
L - J : 9:00–18:00 hs
V : 9:00–15:00 hs